
what is the outer electron configuration for column 5AĮs/91145038_1/cl/outline b.tulsacc.e.How many outer electrons does it have? How many valence electrons does it have? 5 How many core electrons does it have? Consider the electron configuration for vanadium. How many outer electrons does it have? O How many valence electrons does it have? 7 How many core electrons does it have? 46 Enter your answer as an integer value. How many outer electrons does it.Ĭonsider the electron configuration for iodine. ns2npĬonsider the electron configuration for iodine.
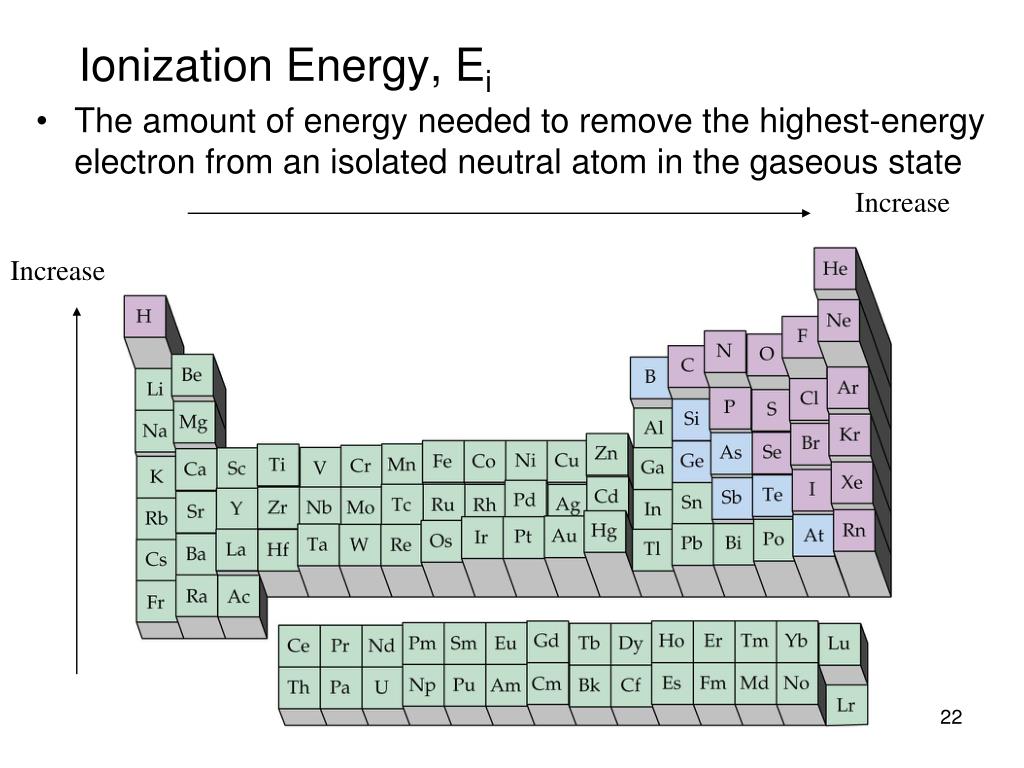
nsnp 54./Which outer electron configurations would you expect to belong to a noble gas? To a metalloi d? b. Which outer electron configuration would you expect to belong to a reactive metal? To a reactive nonmetal? b. Which outer electron configuration would you expect to belong to a reactive.ĥ3.
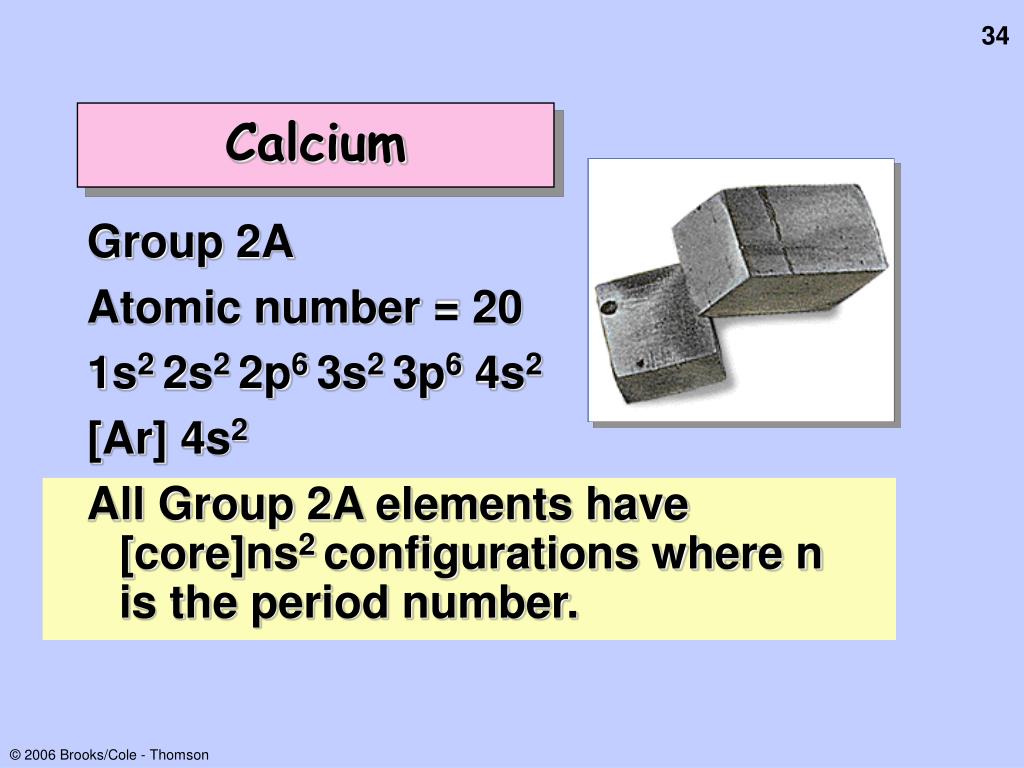
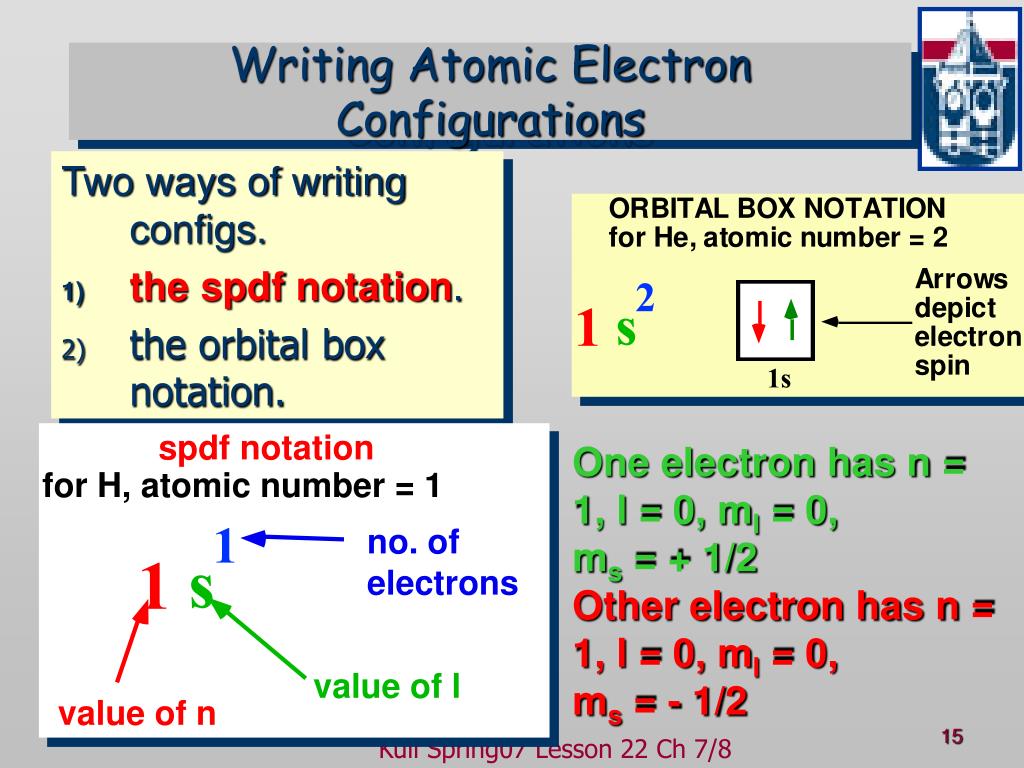
Question DetailsGive the outer electron configuration for each of the following columns in the periodic table.1)3AExpress your answer as a string without blank space between orbitals. Give the outer electron configuration for each of the following columns in the periodic table.Identify the generic outer electron configuration for the alkaline earth metals. Identify the generic outer electron configuration for the alkaline earth metals.


 0 kommentar(er)
0 kommentar(er)
